Introduction to High-Intensity Sweeteners
 Whether you call them sugar substitutes, non-nutritive sweeteners or high-intensity sweeteners, you’re looking at substances that impart a sweet taste to foods and beverages. They do this either without calories or with only a few calories, and they have little to no impact on blood glucose levels.High-intensity sweeteners are much sweeter than sugar and are regulated as food additives by the Food and Drug Administration (FDA). The FDA establishes an acceptable daily intake (ADI) for every approved high-intensity sweetener. The ADI is the amount considered safe for adults to consume every day over the course of an entire lifetime.In addition to the six high-intensity sweeteners that are FDA-approved as food additives, GRAS (a.k.a. generally recognized as safe) notices have been submitted for two sweeteners currently in common use.
Whether you call them sugar substitutes, non-nutritive sweeteners or high-intensity sweeteners, you’re looking at substances that impart a sweet taste to foods and beverages. They do this either without calories or with only a few calories, and they have little to no impact on blood glucose levels.High-intensity sweeteners are much sweeter than sugar and are regulated as food additives by the Food and Drug Administration (FDA). The FDA establishes an acceptable daily intake (ADI) for every approved high-intensity sweetener. The ADI is the amount considered safe for adults to consume every day over the course of an entire lifetime.In addition to the six high-intensity sweeteners that are FDA-approved as food additives, GRAS (a.k.a. generally recognized as safe) notices have been submitted for two sweeteners currently in common use.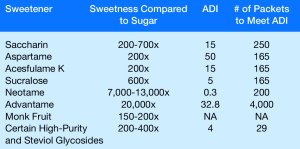 FDA-Approved Sugar SubstitutesSaccharin was first discovered and used in 1879, before the current food additive approval process came into effect in 1958. Brand names include Sweet and Low®, Sweet Twin®, and Necta Sweet®. It is 200 to 700 times sweeter than table sugar and contains no calories.In the early 1970’s saccharin was linked to bladder cancer in lab rats, which led to a warning label on saccharin-containing foods until scientific research could establish safety. Since then, more than 30 human studies demonstrated that saccharin is safe for human use, and that the association with cancer found in rats does not happen in humans. Foods containing saccharin no longer are required to carry the warning label.Saccharin is currently approved for use in beverages, fruit juice drinks, as a sugar substitute for cooking or table use, and in processed foods.Aspartame was first approved for use in 1981. Brand names include Nutrasweet®, Equal®, and Sugar Twin®. Aspartame is made from two amino acids, phenylalanine and aspartic acid. People with a rare hereditary disease known as phenylketonuria (PKU) have a difficult time metabolizing phenylalanine, and must control their intake of phenylalanine from all sources, including aspartame.Although it contains 4 calories per gram of weight, aspartame is 200 times sweeter than sugar and only very small amounts need to be used so that the calorie contribution is almost zero. Long exposure to heat causes aspartame to break down, reducing its sweetening power. Therefore, it is not recommended for use in baking or in cooking methods that require extended exposure to high temperatures.
FDA-Approved Sugar SubstitutesSaccharin was first discovered and used in 1879, before the current food additive approval process came into effect in 1958. Brand names include Sweet and Low®, Sweet Twin®, and Necta Sweet®. It is 200 to 700 times sweeter than table sugar and contains no calories.In the early 1970’s saccharin was linked to bladder cancer in lab rats, which led to a warning label on saccharin-containing foods until scientific research could establish safety. Since then, more than 30 human studies demonstrated that saccharin is safe for human use, and that the association with cancer found in rats does not happen in humans. Foods containing saccharin no longer are required to carry the warning label.Saccharin is currently approved for use in beverages, fruit juice drinks, as a sugar substitute for cooking or table use, and in processed foods.Aspartame was first approved for use in 1981. Brand names include Nutrasweet®, Equal®, and Sugar Twin®. Aspartame is made from two amino acids, phenylalanine and aspartic acid. People with a rare hereditary disease known as phenylketonuria (PKU) have a difficult time metabolizing phenylalanine, and must control their intake of phenylalanine from all sources, including aspartame.Although it contains 4 calories per gram of weight, aspartame is 200 times sweeter than sugar and only very small amounts need to be used so that the calorie contribution is almost zero. Long exposure to heat causes aspartame to break down, reducing its sweetening power. Therefore, it is not recommended for use in baking or in cooking methods that require extended exposure to high temperatures. Acesulfame potassium was first approved for use in 1988. It’s also known as acesulfame K or Ace-K because ‘K’ is the chemical shorthand for potassium. Brand names include Sunett® and Sweet One®. It is about 200 times sweeter than sugar and is often combined with other sweeteners. Acesulfmae K is heat stable and typically used in frozen desserts, candies, beverages, and baked goods.Sucralose was first approved for use in 1998. Commonly known as Splenda, it is 600 times sweeter than sugar. Sucralose is heat stable and was developed from a sugar molecule. It has been used in a variety of foods including baked goods, beverages, chewing gum, gelatins, and frozen dairy desserts.Neotame was approved in 2002. The brand name is Newtame, and it is 7,000-13,000 times sweeter than sugar. Neotame is made from a combination of phenylalanine and aspartic acid. Because it is so much sweeter than sugar, the amount of phenylalanine present is very low and does not pose a risk to people with PKU. Neotame is typically used in combination with other high-intensity sweeteners and is not available as a tabletop sweetener.Advantame is the newest high-intensity sweetener, approved in 2014. It does not yet have a brand name. Advantame is heat stable and 20,000 times sweeter than sugar. Since advantame is so much sweeter than aspartame, only a tiny amount is used to produce a similar level of sweetness, and this amount does not contain significant amounts of phenylalanine and is safe for people with PKU.Advantame has been approved by the FDA for use as a general-purpose sweetener and flavor enhancer and can be used in baked goods, non-alcoholic beverages (including soft drinks), chewing gum, confections and frostings, frozen desserts, gelatins and puddings, jams and jellies, processed fruits and fruit juices, toppings, and syrups.GRAS High-Intensity SweetenersWhole stevia leaves contain a number of active components, not all of them sweet. The leaves are considered supplements, and are not permitted to be sold within the United States as sweeteners. In 2008 the FDA allowed GRAS status for purified stevia components rebaudioside A and stevioside that provide a sweet taste 200-400 times sweeter than sugar. Brand names include Truvia®, PureVia®, and Enliten®.Monk fruit extract or luo han guo is 150-300 times sweeter than table sugar, and was approved for GRAS status in 2010. Brand names include Nectresse®, Monk Fruit in the Raw®, and PureLo®.By Lynn Grieger RDN, CDE, CPT, CWCReferences
Acesulfame potassium was first approved for use in 1988. It’s also known as acesulfame K or Ace-K because ‘K’ is the chemical shorthand for potassium. Brand names include Sunett® and Sweet One®. It is about 200 times sweeter than sugar and is often combined with other sweeteners. Acesulfmae K is heat stable and typically used in frozen desserts, candies, beverages, and baked goods.Sucralose was first approved for use in 1998. Commonly known as Splenda, it is 600 times sweeter than sugar. Sucralose is heat stable and was developed from a sugar molecule. It has been used in a variety of foods including baked goods, beverages, chewing gum, gelatins, and frozen dairy desserts.Neotame was approved in 2002. The brand name is Newtame, and it is 7,000-13,000 times sweeter than sugar. Neotame is made from a combination of phenylalanine and aspartic acid. Because it is so much sweeter than sugar, the amount of phenylalanine present is very low and does not pose a risk to people with PKU. Neotame is typically used in combination with other high-intensity sweeteners and is not available as a tabletop sweetener.Advantame is the newest high-intensity sweetener, approved in 2014. It does not yet have a brand name. Advantame is heat stable and 20,000 times sweeter than sugar. Since advantame is so much sweeter than aspartame, only a tiny amount is used to produce a similar level of sweetness, and this amount does not contain significant amounts of phenylalanine and is safe for people with PKU.Advantame has been approved by the FDA for use as a general-purpose sweetener and flavor enhancer and can be used in baked goods, non-alcoholic beverages (including soft drinks), chewing gum, confections and frostings, frozen desserts, gelatins and puddings, jams and jellies, processed fruits and fruit juices, toppings, and syrups.GRAS High-Intensity SweetenersWhole stevia leaves contain a number of active components, not all of them sweet. The leaves are considered supplements, and are not permitted to be sold within the United States as sweeteners. In 2008 the FDA allowed GRAS status for purified stevia components rebaudioside A and stevioside that provide a sweet taste 200-400 times sweeter than sugar. Brand names include Truvia®, PureVia®, and Enliten®.Monk fruit extract or luo han guo is 150-300 times sweeter than table sugar, and was approved for GRAS status in 2010. Brand names include Nectresse®, Monk Fruit in the Raw®, and PureLo®.By Lynn Grieger RDN, CDE, CPT, CWCReferences
- High Intensity Sweeteners. US Food and Drug Administration. http://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm397716.htm Last updated 7-9-14. Accessed 8-11-14
- Use of nutritive and non-nutritive sweeteners. Position of the Academy of Nutrition and Dietetics. J Acad Nutr Diet. 2012 May;112(5):739-58
- Everything You Need to Know About Aspartame. International Food Information Council. http://www.foodinsight.org/Content/3848/FINAL_Aspartame%20Brochure_Web%20Version_11-2011.pdf August 2011. Accessed 8-11-14.
- Advantame. US Food and Drug Administration. http://www.fda.gov/Food/NewsEvents/ConstituentUpdates/ucm397740.htm Last updated 6-23-14. Accessed 8-11-14.